RSV Vaccine Approved by FDA: A Breakthrough
We are excited to announce the recent RSV vaccine approved by FDA. This vaccine, which is developed by Pfizer, offers hope for the prevention of respiratory syncytial virus (RSV) in infants and adults alike. As a trusted source of health information, we are committed to providing comprehensive and up-to-date information about this vaccine and its potential benefits. Continue reading to learn about this breakthrough news!
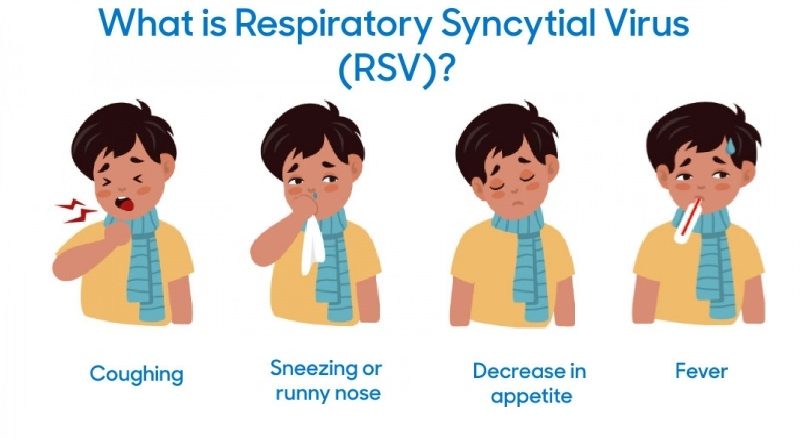
What is RSV?
Respiratory syncytial virus (RSV) is also called human respiratory syncytial virus (hRSV) and human orthopneumovirus. It is a common virus that affects the respiratory system. It’s so common that most children have been infected with the virus by age 2. It can cause mild to severe illness, especially in infants, older adults, and people with weakened immune systems. Symptoms can range from a runny nose and cough to more serious conditions such as bronchiolitis or pneumonia.
RSV is highly contagious and can be spread through contact with respiratory secretions such as saliva or mucus from infected individuals. It can also be spread by touching contaminated surfaces and then touching your mouth, nose, or eyes. A transmission way that is common for all respiratory diseases.
Why is the RSV vaccine important?
The RSV vaccine is an important development in the prevention of this highly contagious virus. Infants and young children, especially those with underlying health conditions, are at a higher risk of developing severe RSV illness, which can lead to hospitalization and even death. The vaccine offers protection against this disease in this vulnerable population.
The RSV vaccine is also important for older adults, especially those with chronic health conditions such as heart disease or lung disease. These individuals are at a higher risk of developing severe RSV illness and can benefit from the protection offered by the vaccine.
How does the RSV vaccine work?
It works by triggering an immune response to the virus. The vaccine contains a protein found on the surface of the RSV virus, which stimulates the body’s immune system to produce antibodies against the virus. These antibodies help protect against future infections.
The vaccine is given in two doses, spaced one month apart. It can be given to infants as young as six months old, as well as older adults.
Side effects of the RSV vaccine
Like any vaccine, the RSV vaccine has some side effects, of which the most common are mild. It includes pain or swelling at the injection site, fever, and fussiness. These side effects typically resolve on their own within a few days.
In rare cases, more serious side effects can occur including allergic reactions or seizures. However, the benefits of the vaccine in preventing severe RSV illness far outweigh the risks of these rare side effects.
SUMMARY
The FDA has recently approved the world’s first RSV vaccine, which is a breakthrough development in the prevention of this highly contagious virus. The vaccine offers protection for infants, young children, and older adults at risk of severe RSV illness. It works by triggering an immune response to the virus and is given in two doses. Like all vaccines, there may be mild side effects. However, the benefits of the vaccine in preventing severe RSV illness far outweigh its risk. Protect yourself and your loved ones by staying informed and getting vaccinated at the correct time.